Structural systems biology
Protein conformational changes are an integral part of numerous cellular processes. Yet many structural methods study proteins in isolated form, outside of the crowded and dynamic environment of the cell. To address this issue, the Picotti laboratory has pioneered LiP-MS (limited proteolysis-mass spectrometry), in which protein conformational changes can be monitored within the complex cellular milieu. In LiP-MS, regions of a protein inaccessible to protease are detected and quantitatively measured by mass spectrometry; changes in LiP profiles in varying conditions then give a picture of structural changes. This approach is both deep and broad: it has peptide-level resolution, but simultaneously monitors structural changes across the proteome. LiP-MS detects structural changes due to various molecular events, including protein-protein binding, allostery, and aggregation.
In the laboratory, we continue to advance this approach as well as its applications, aiming continuously closer to a systems view of protein structural dynamics.
Selected Publications

V. Cappelletti, T. Hauser, I. Piazza, M. Pepelnjak, L. Malinovska, T. Fuhrer, Y. Li, C. Dörig, P. Boersema, L. Gillet, J. Grossbach, A. Dugourd, J. Saez-Rodriguez, A. Beyer, N. Zamboni, A. Caflisch, N. de Souza, P. Picotti. external page Dynamic 3D proteomes reveal protein functional alterations at high resolution in situ. (2021) Cell, 184 (2), 545-559. e22.
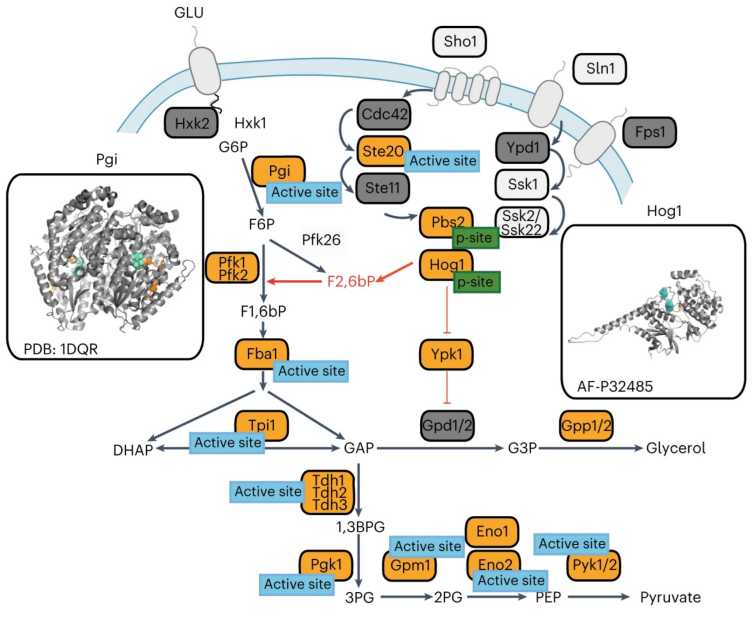
L. Malinovska, V. Cappelletti, D. Kohler, I. Piazza, T.H. Tsai, M. Pepelnjak, P. Stalder, C. Dörig, F. Sesterhenn, F. Elsässer, Kralickova L., Beaton N., Reiter L., N. de Souza, O. Vitek, P. Picotti. external page Proteome-wide structural changes measured with limited proteolysis-mass spectrometry: an advanced protocol for high-throughput applications. (2023) Nat Protoc., 18(3):659-682.

S. Schopper, A. Kahraman, P. Leuenberger, Y. Feng, I. Piazza, O. Müller, P.J. Boersema, P. Picotti. external page Measuring protein structural changes on a proteome-wide scale using limited proteolysis-coupled mass spectrometry. (2017) Nat. Protoc.., 12 (11), 2391-2410.
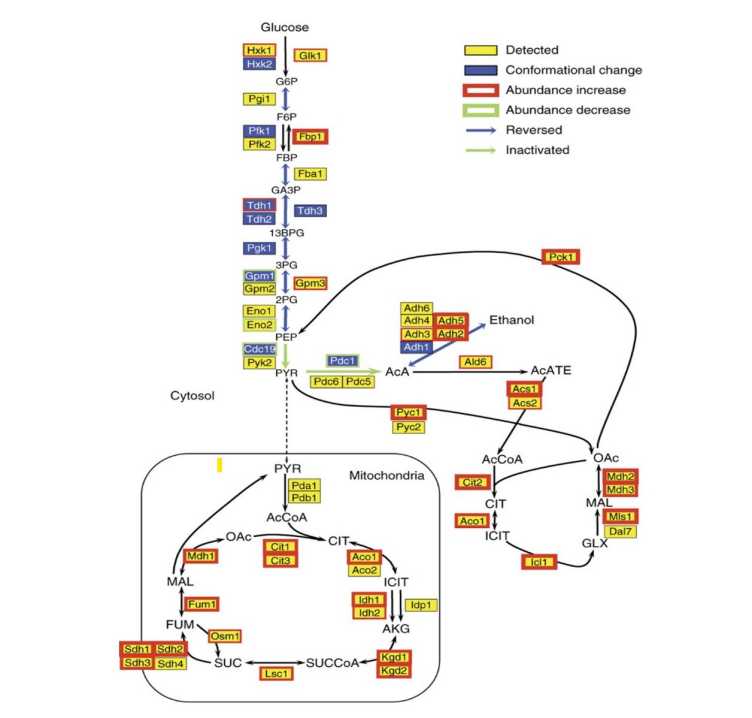
Y. Feng, G. De Franceschi, A. Kahraman, M. Soste, A. Melnik, P.J. Boersema, P.P. de Laureto, Y. Nikolaev, A.P. Oliveira, P. Picotti. external page Global analysis of protein structural changes in complex proteomes. (2014) Nat. Biotechnol., 32 (10), 1036-1044.
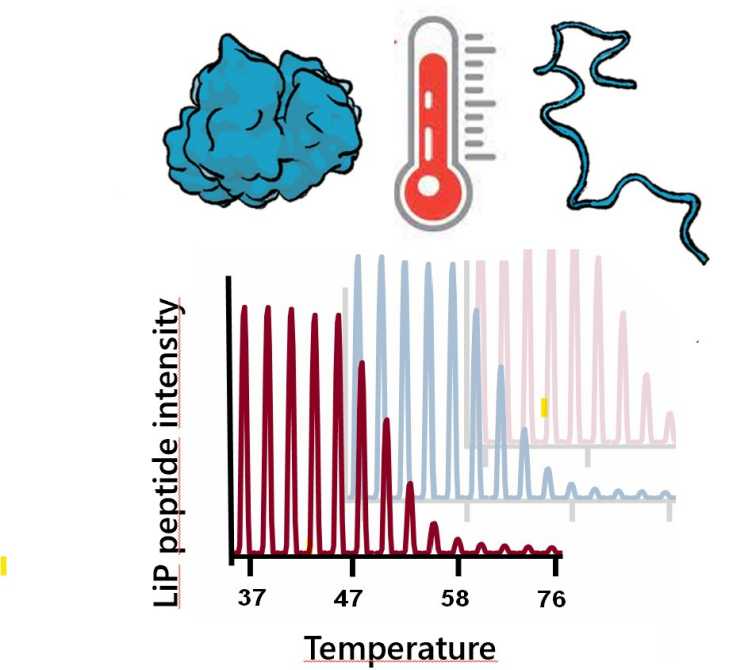
P. Leuenberger, S. Ganscha, A. Kahraman, V. Cappelletti, P.J. Boersema, C. von Mering, M. Claassen, P. Picotti. external page Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability. (2017) Science, 355 (6327), eaai7825.