Our study on the determinants of proteome thermostability was just published in Science!
Our paper entitled "Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability" has just been published in Science.
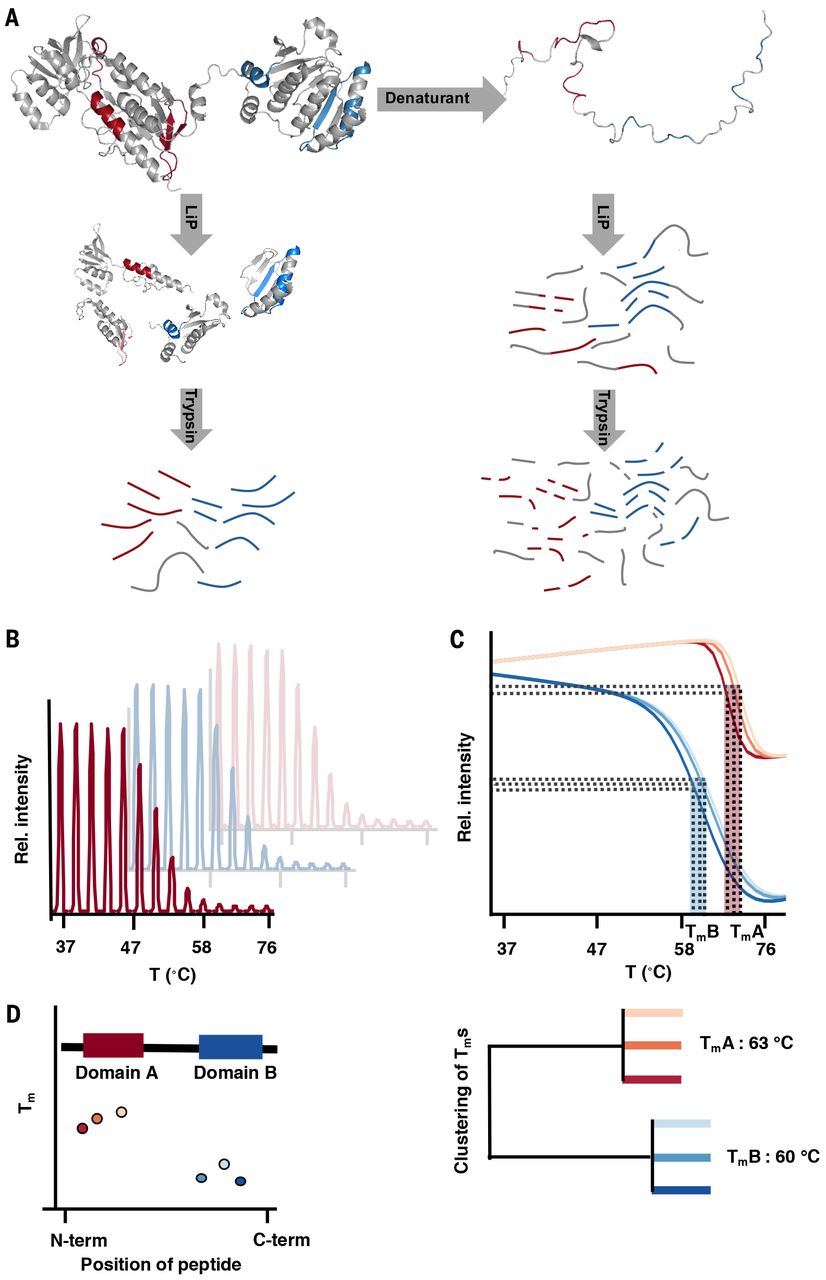
In this study, we used a structural proteomic approach based on limited proteolysis to analyze protein thermostability on a proteome-wide scale and with domain-level resolution. We applied our strategy to the proteomes of Escherichia coli, Saccharomyces cerevisiae, the thermophilic bacterium Thermus thermophilus, and human cells, yielding thermostability data for more than 8,000 proteins and about 20,000 protein domains. Our data indicate that temperature-induced cellular collapse is due to the loss of a subset of unstable proteins with key physiological functions, reveal the molecular and biological bases of protein thermal stability, and indicate that the fraction of intrinsically disordered proteins in a cell is far lower than initially predicted. Further, our findings suggest that over the course of evolution, the burden of intracellular misfolding has been reduced by increasing the thermodynamic stability of abundant proteins.