Determinants of proteome thermostability

Every biological organism thrives within an optimal range of temperature, outside of which it is no longer viable. Proteins are thought to be the key macromolecules that respond to extremes of temperature, since they are typically of lower stability and present at lower abundance than other macromolecular players.
We use a structural proteomic approach to study the determinants of protein thermal stability. Our approach enables monitoring protein thermal denaturation profiles directly in complex cell extracts and across the proteome. We have recently examined proteome thermal stability in several organisms. We identified a key subset of essential proteins denatures at the temperature of thermal cell death in E. coli. In thermophilic bacteria living in hot sprigs these proteins are stabilized and the proteome is globally less prone to aggregation. We continue to investigate this fundamental biophysical properties, extending our studies to the mechanism of thermoprotectants.
Selected Publications

M. Pepelnjak, B. Velten, N. Näpflin, T. von Rosen, U.C. Palmiero, J.H. Ko, H.D. Maynard, P. Arosio, E. Weber-Ban, N. de Souza, W. Huber, P. Picotti. external page In situ analysis of osmolyte mechanisms of proteome thermal stabilization. (2024) Nat. Chem. Biol., Feb 29. 2024
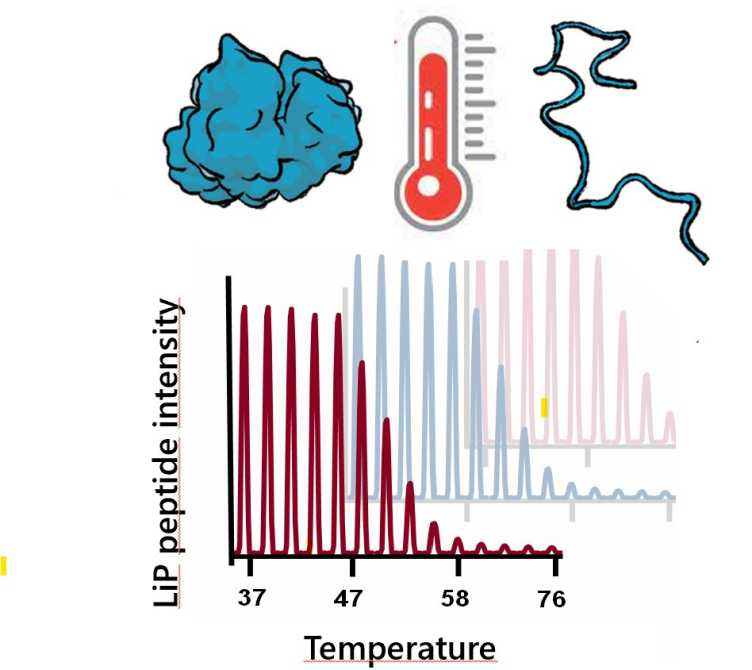
P. Leuenberger, S. Ganscha, A. Kahraman, V. Cappelletti, P.J. Boersema, C. von Mering, M. Claassen, P. Picotti. external page Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability. (2017) Science, 355 (6327), eaai7825.